Ready to transform your health?
Unlock access to expert guidance and a weight care plan crafted just for you.
Similar Articles
Similar Articles

How to Spot Fake Semaglutide: Safety Risks and Warning Signs
How to Spot Fake Semaglutide: Safety Risks and Warning Signs

Alternatives to BMI: Better Ways to Measure Health
Alternatives to BMI: Better Ways to Measure Health

Is Zofran Safe with Semaglutide? What You Need to Know
Is Zofran Safe with Semaglutide? What You Need to Know
CBL-514: A New Injectable Approach to Targeted Fat Reduction
Learn about CBL-514, an investigational injectable treatment for localized abdominal fat reduction. Understand Phase 2 trial results, how it works, and what makes it different from systemic weight loss medications.
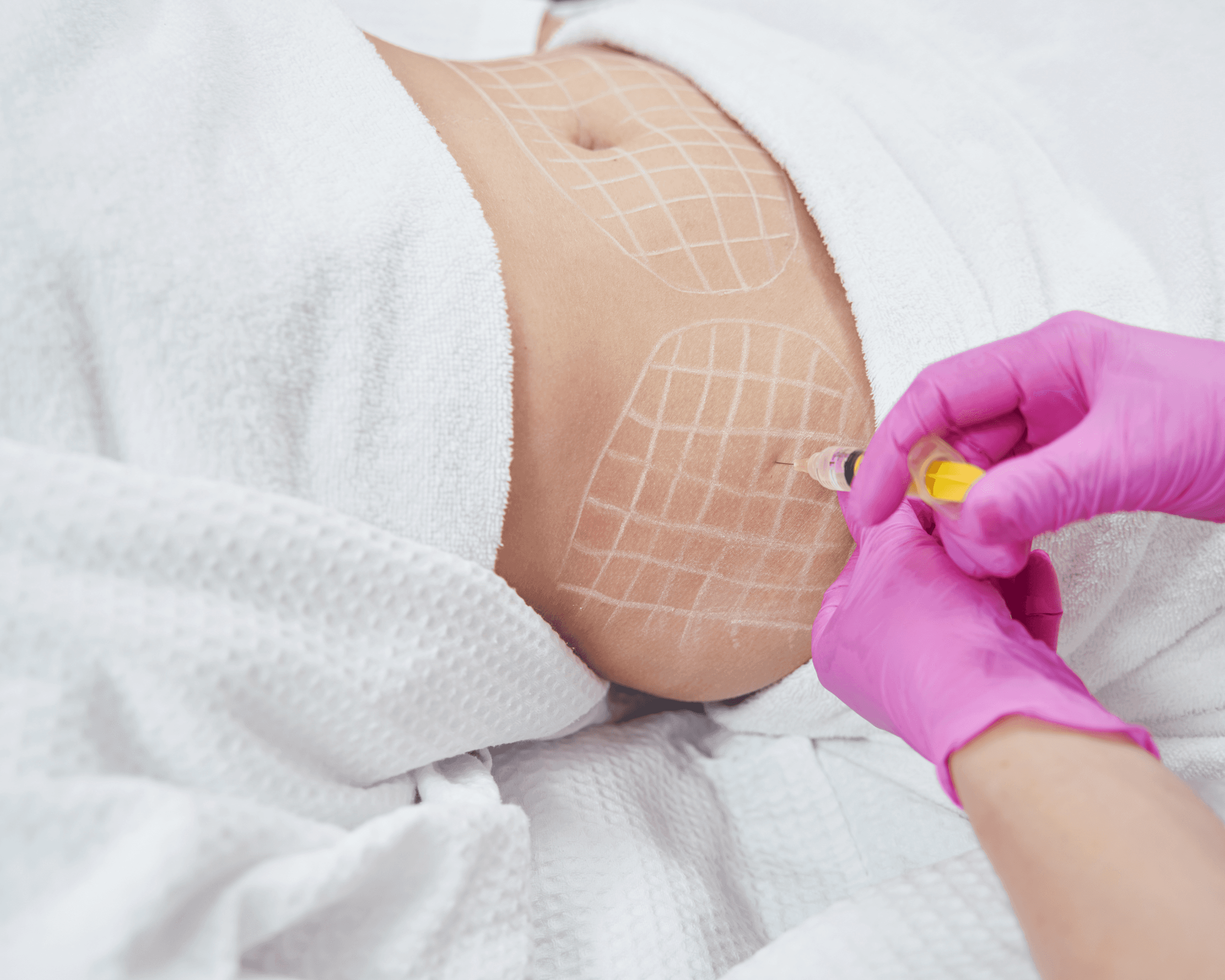
Table of Contents
Table of Contents
What is CBL-514 and How Does It Work?
Phase 2 Trial Design and Methods
Phase 2 Trial Effectiveness
Phase 2 Trial Safety Profile
How CBL-514 Compares to Other Fat Reduction Options
Who Might Be Candidates for CBL-514
Current Status and What Comes Next
The Relationship to Systemic Weight Loss
References
What is CBL-514 and How Does It Work?
Phase 2 Trial Design and Methods
Phase 2 Trial Effectiveness
Phase 2 Trial Safety Profile
How CBL-514 Compares to Other Fat Reduction Options
Who Might Be Candidates for CBL-514
Current Status and What Comes Next
The Relationship to Systemic Weight Loss
References
Body contouring and fat reduction occupy a space between systemic weight loss (which affects your entire body) and invasive surgery like liposuction. You can lose significant weight with medications like GLP-1 receptor agonists or through diet and exercise, but these approaches do not selectively target specific areas of stubborn fat. Conversely, liposuction effectively removes localized fat but requires surgery, anesthesia, recovery time, and carries surgical risks.
For years, the middle ground has been limited. Treatments like cryolipolysis (fat freezing) and various energy-based devices offer non-surgical options, but results vary and multiple expensive sessions are often needed. The only FDA-approved injectable for fat reduction is deoxycholic acid (Kybella), which works only for submental fat (double chin) and has notable side effects including potential nerve damage.
CBL-514 represents a different approach: an injectable small-molecule drug designed to induce targeted fat cell death (apoptosis) in larger treatment areas like the abdomen. Published Phase 2 trial results in March 2025 showed that nearly 70% of participants achieved clinically meaningful fat reduction with up to four treatments, with some seeing results after just one injection session. The safety profile appeared favorable with primarily mild to moderate injection site reactions and no serious systemic effects.
This article examines what CBL-514 is and how it works, Phase 2 clinical trial results showing effectiveness and safety, how it compares to other fat reduction options, who might be candidates for this treatment, and what comes next as the medication moves toward Phase 3 trials.
What Is CBL-514 and How Does It Work?
CBL-514 is an investigational small-molecule drug administered through subcutaneous injections directly into areas of unwanted fat. Unlike systemic weight loss medications that work throughout your entire body, CBL-514 works locally at the injection site to reduce fat in targeted areas.
The mechanism involves inducing apoptosis (programmed cell death) specifically in adipocytes (fat cells). Preclinical studies showed that CBL-514 upregulates apoptosis mediators including caspase 3 and increases the Bax/Bcl-2 ratio, triggering fat cells to undergo controlled death. This is different from necrosis (uncontrolled cell death that causes inflammation and tissue damage). Apoptosis allows fat cells to die in an organized way that the body then clears through normal processes.
The key distinguishing feature is that CBL-514 appears to selectively affect adipocytes without causing necrosis, skin ulceration, nerve damage, or systemic effects on major body systems based on preclinical studies. Animal toxicology studies found no damage to organs or tissues surrounding the injection site.
After injection into subcutaneous fat, the medication induces apoptosis in fat cells within the treated area. The body's natural clearance mechanisms then remove the dead cells and released fat over the following weeks. This results in measurable reduction in fat volume and thickness in the injection area.
Important context: CBL-514 is investigational and not FDA-approved. The medication has completed Phase 2 trials with results published in March 2025. Caliway Biopharmaceuticals plans to begin Phase 3 trials in late 2025. If successful, regulatory approval could potentially occur in 2026 or later, though timelines are uncertain. The medication is not currently available outside clinical trials.
Phase 2 Trial Design and Methods
The Phase 2 trial published in Aesthetic Surgery Journal in March 2025 provides the most comprehensive evidence available for CBL-514 effectiveness and safety.
This was a randomized, single-blind, placebo-controlled, parallel-group study conducted from February 2022 to March 2023 at five centers in the United States and Australia. The trial enrolled 76 participants who were randomized 2:1 to receive either CBL-514 or placebo injections.
Participants were adults aged 18 to 64 with BMI between 18.5 and 35 kg/m² and abdominal skinfold thickness of 3 to 8 cm measured by caliper. The study specifically targeted people with localized abdominal fat suitable for reduction, not people with obesity requiring systemic weight loss.
Treatment involved up to four sessions spaced four weeks apart. Each treatment session consisted of 25 to 50 subcutaneous injections into the abdominal area, with each injection delivering 2.4 mL of CBL-514 at a concentration of 2 mg/cm². The maximum dose per treatment was 600 mg. Injections were spaced 2.45 cm apart across the treatment area.
Follow-up visits occurred at 4 and 8 weeks after the final treatment. Subcutaneous fat thickness was measured using ultrasound at four scan points on the abdomen. Fat volume was calculated using these thickness measurements over a defined treatment area of 300 cm². The study also used 3D body imaging to visualize changes.
The primary endpoint was the proportion of participants achieving at least 150 mL subcutaneous fat volume reduction from baseline. Secondary endpoints included proportion achieving at least 200 mL reduction, number of treatments needed, and total fat volume change.
Phase 2 Trial Effectiveness
The trial met its primary endpoint and all secondary endpoints, showing statistically significant fat reduction with CBL-514 compared to placebo.
At the 8-week follow-up (8 weeks after final treatment), 69.6% of CBL-514-treated participants achieved at least 150 mL subcutaneous fat volume reduction, compared to 0% of placebo-treated participants. This difference was highly statistically significant.
For the higher threshold of 200 mL reduction, 60.9% of CBL-514 participants achieved this target at 8-week follow-up, compared to 0% of placebo participants.
The average fat volume reduction was substantial. CBL-514-treated participants showed a mean change in fat volume of approximately 210 mL compared to baseline, which was over 200 mL more than the placebo group. In the per-protocol population (participants who completed all treatments and follow-ups with stable body weight), the mean fat volume reduction exceeded 300 mL.
Importantly, some participants responded to very limited treatment. Of the 28 CBL-514 participants who achieved at least 150 mL fat reduction, 42.9% (12 participants) reached this threshold after just one treatment session. Another 35.7% required two treatments. This means over 75% of responders achieved clinically meaningful results with one or two treatment sessions.
The largest fat volume reduction recorded was over 750 mL in a single CBL-514-treated participant. These reductions occurred with minimal changes in overall body weight. The average body weight change was less than 1 kg in both treatment groups, confirming that fat reduction was localized to the injection area rather than reflecting systemic weight loss.
Representative photographs showed visible reduction in lower abdominal contour, with ultrasound imaging confirming decreased fat layer thickness across measurement points. Notably, participants did not exhibit significant skin laxity after fat reduction, suggesting the skin adapted well to the volume change.
Phase 2 Trial Safety Profile
Safety data from the Phase 2 trial provides important information about the tolerability of CBL-514 treatment.
Nearly all participants (99%) experienced at least one treatment-related adverse event. However, almost all of these were injection site reactions, which is expected with any injectable treatment. The most common adverse events were injection site erythema (redness) in 93.9% of CBL-514 participants, injection site pain in 93.9%, injection site pruritus (itching) in 85.7%, injection site swelling in 83.7%, and injection site warmth in 83.7%.
The severity profile was generally favorable. All participants experienced mild reactions. About half (49%) experienced moderate reactions. Only 9 participants (18.4%) reported severe reactions, all of which involved injection site pain. No placebo participants reported severe reactions.
For participants who experienced injection site pain (63 total across both groups), 60.3% required medication for relief, primarily over-the-counter pain relievers. For the majority (90.5%), pain resolved within one week.
Only two participants discontinued treatment due to adverse events: one due to injection site pain and one due to injection site discoloration. One serious adverse event (peritoneal metastasis, a cancer-related finding) occurred in a CBL-514 participant but was deemed unrelated to study treatment.
Importantly, the severe adverse events associated with deoxycholic acid injections (skin necrosis, ulceration, nerve injury) were notably absent in this trial. Laboratory tests, vital signs, ECGs, and physical examinations showed no clinically significant abnormalities attributed to CBL-514 treatment, suggesting the medication acts locally without systemic effects.
Two participants with pre-existing high cholesterol showed elevated lipid levels at one study visit, with one reporting hyperlipidemia possibly related to treatment. This may reflect fat metabolism after lipolysis, though the small numbers make it difficult to draw conclusions.
How CBL-514 Compares to Other Fat Reduction Options
Understanding how CBL-514 stacks up against existing options helps contextualize what this treatment might offer if it receives approval.
Versus Liposuction
Liposuction remains the gold standard for targeted fat removal, capable of removing large volumes (liters) of fat in a single procedure. However, it requires surgery, anesthesia, significant recovery time, and carries risks including infection, bleeding, contour irregularities, and rare but serious complications.
The Phase 2 trial authors noted that fat volume reductions with CBL-514 were "comparable to that achieved with liposuction," though this claim requires context. Liposuction typically removes much larger volumes than the 200 to 300 mL average seen with CBL-514. However, for people seeking modest fat reduction in targeted areas without surgery, CBL-514 might offer meaningful improvement without surgical risks.
Versus Cryolipolysis (CoolSculpting)
Cryolipolysis is FDA-approved for fat reduction in the abdomen and flanks. Studies have shown varying results, with one reporting average fat volume reduction of 264.8 mL in the abdomen and flanks at 12 weeks after up to 24 treatment cycles over two sessions. Another study showed 39.6 mL reduction in the flank after a single cycle.
Direct comparison is difficult because measurement techniques differ across studies. However, CBL-514 appears to achieve similar or potentially greater fat reduction per treatment session based on available data. The advantage of cryolipolysis is that it requires no injections, while CBL-514 requires dozens of injection points per treatment.
Versus Deoxycholic Acid (Kybella)
Deoxycholic acid is FDA-approved only for submental fat (double chin). It works by disrupting fat cell membranes, causing cell death and subsequent absorption. Multiple treatment sessions are typically needed, and side effects can be significant including nerve damage risk in the submental area.
CBL-514 is designed for larger body areas where deoxycholic acid has not been extensively tested. The mechanism (apoptosis versus membrane disruption) appears to offer a safety advantage based on Phase 2 data, though head-to-head comparisons do not exist.
Who Might Be Candidates for CBL-514?
Based on the Phase 2 trial enrollment criteria and results, potential candidates would include adults who are not significantly overweight (BMI under 35) but have localized abdominal fat that bothers them, people who have achieved weight loss through diet, exercise, or medications but have remaining stubborn fat deposits, individuals seeking fat reduction without surgery or general anesthesia, and people willing to undergo multiple injection treatments for targeted results.
CBL-514 is designed for localized fat reduction, not treatment of obesity. If you need to lose significant overall weight, systemic approaches like GLP-1 medications combined with lifestyle changes would be more appropriate. CBL-514 addresses remaining fat deposits after weight has been lost, or treats localized fat in people who are otherwise at healthy weight.
The trial required participants to maintain stable weight during treatment. This suggests CBL-514 works best when you are not actively gaining or losing weight systemically but want to address specific areas.
Current Status and What Comes Next
CBL-514 is investigational, meaning it is not approved for use outside clinical trials. Caliway Biopharmaceuticals completed Phase 2 trials in 2023 to 2025 and announced plans to begin Phase 3 trials in late 2025.
Phase 3 trials will likely enroll larger numbers of participants, possibly hundreds across multiple sites. These trials will need to confirm the Phase 2 efficacy findings in a larger population and gather more extensive safety data over longer follow-up periods. Regulatory agencies require Phase 3 data before considering approval.
If Phase 3 trials are successful, Caliway would submit for FDA approval, likely in 2026 or 2027. The regulatory review process typically takes 6 to 12 months or longer. Realistically, CBL-514 is unlikely to be commercially available before 2027 at the earliest, and could take longer depending on trial results and regulatory review.
Pricing is unknown. The treatment would likely require a prescription if approved. Insurance coverage for cosmetic fat reduction procedures is generally excluded, suggesting this would be an out-of-pocket expense if it reaches market.
The Relationship to Systemic Weight Loss
It is important to distinguish between systemic weight loss and localized fat reduction. These address different concerns and serve different patient populations.
Systemic weight loss through GLP-1 medications like semaglutide and tirzepatide reduces fat throughout your body by decreasing appetite and calorie intake. You lose weight globally, with reductions in visceral fat (around organs), subcutaneous fat (under skin), and improvements in metabolic health. These medications help with overall weight management and associated health conditions.
Localized fat reduction through injectable treatments like CBL-514 (if approved) targets specific areas of stubborn subcutaneous fat without affecting overall body weight significantly. The Phase 2 trial showed minimal body weight changes despite measurable fat volume reductions in the treatment area.
Some people might benefit from both approaches sequentially. Achieving overall weight loss through systemic approaches first, then addressing remaining localized fat deposits with targeted treatments, represents a logical progression. However, these are different tools serving different purposes.
At Mochi Health, we focus on comprehensive weight management through GLP-1 medications, nutrition counseling, and lifestyle support. While we don't currently offer injectable fat reduction treatments like CBL-514, we help patients achieve significant systemic weight loss that improves overall health. For people interested in learning about weight management options currently available, visit https://joinmochi.com/medications.
Check Your Eligibility
If you want to learn whether GLP-1 treatment is right for you and receive personalized guidance from providers who understand comprehensive weight management, you can start by completing Mochi's eligibility questionnaire. Check your eligibility here: https://app.joinmochi.com/eligibility.
References
Gold, M., Schlessinger, J., Goodman, G. J., Dayan, S., DuBois, J., Ling, Y. F., Sheu, A. Y., Ho, W. W. S., & Chou, Y. C. (2025). Efficacy and safety of CBL-514 injection in reducing abdominal subcutaneous fat: A randomized, single-blind, placebo-controlled phase II study. Aesthetic Surgery Journal, 45(6), 611-620. https://doi.org/10.1093/asj/sjaf032
Shome, D., Khare, S., & Vadera, S. (2022). Efficacy of a novel injection lipolysis to induce targeted adipocyte apoptosis: A randomized, phase IIa study of CBL-514 injection on abdominal subcutaneous fat reduction. Aesthetic Surgery Journal, 42(10), 1154-1164. https://doi.org/10.1093/asj/sjac087
This article is for educational purposes only and should not be considered medical advice. CBL-514 is an investigational medication not currently FDA-approved or commercially available. Consult with healthcare providers about currently available options for fat reduction and weight management appropriate for your individual needs.
Body contouring and fat reduction occupy a space between systemic weight loss (which affects your entire body) and invasive surgery like liposuction. You can lose significant weight with medications like GLP-1 receptor agonists or through diet and exercise, but these approaches do not selectively target specific areas of stubborn fat. Conversely, liposuction effectively removes localized fat but requires surgery, anesthesia, recovery time, and carries surgical risks.
For years, the middle ground has been limited. Treatments like cryolipolysis (fat freezing) and various energy-based devices offer non-surgical options, but results vary and multiple expensive sessions are often needed. The only FDA-approved injectable for fat reduction is deoxycholic acid (Kybella), which works only for submental fat (double chin) and has notable side effects including potential nerve damage.
CBL-514 represents a different approach: an injectable small-molecule drug designed to induce targeted fat cell death (apoptosis) in larger treatment areas like the abdomen. Published Phase 2 trial results in March 2025 showed that nearly 70% of participants achieved clinically meaningful fat reduction with up to four treatments, with some seeing results after just one injection session. The safety profile appeared favorable with primarily mild to moderate injection site reactions and no serious systemic effects.
This article examines what CBL-514 is and how it works, Phase 2 clinical trial results showing effectiveness and safety, how it compares to other fat reduction options, who might be candidates for this treatment, and what comes next as the medication moves toward Phase 3 trials.
What Is CBL-514 and How Does It Work?
CBL-514 is an investigational small-molecule drug administered through subcutaneous injections directly into areas of unwanted fat. Unlike systemic weight loss medications that work throughout your entire body, CBL-514 works locally at the injection site to reduce fat in targeted areas.
The mechanism involves inducing apoptosis (programmed cell death) specifically in adipocytes (fat cells). Preclinical studies showed that CBL-514 upregulates apoptosis mediators including caspase 3 and increases the Bax/Bcl-2 ratio, triggering fat cells to undergo controlled death. This is different from necrosis (uncontrolled cell death that causes inflammation and tissue damage). Apoptosis allows fat cells to die in an organized way that the body then clears through normal processes.
The key distinguishing feature is that CBL-514 appears to selectively affect adipocytes without causing necrosis, skin ulceration, nerve damage, or systemic effects on major body systems based on preclinical studies. Animal toxicology studies found no damage to organs or tissues surrounding the injection site.
After injection into subcutaneous fat, the medication induces apoptosis in fat cells within the treated area. The body's natural clearance mechanisms then remove the dead cells and released fat over the following weeks. This results in measurable reduction in fat volume and thickness in the injection area.
Important context: CBL-514 is investigational and not FDA-approved. The medication has completed Phase 2 trials with results published in March 2025. Caliway Biopharmaceuticals plans to begin Phase 3 trials in late 2025. If successful, regulatory approval could potentially occur in 2026 or later, though timelines are uncertain. The medication is not currently available outside clinical trials.
Phase 2 Trial Design and Methods
The Phase 2 trial published in Aesthetic Surgery Journal in March 2025 provides the most comprehensive evidence available for CBL-514 effectiveness and safety.
This was a randomized, single-blind, placebo-controlled, parallel-group study conducted from February 2022 to March 2023 at five centers in the United States and Australia. The trial enrolled 76 participants who were randomized 2:1 to receive either CBL-514 or placebo injections.
Participants were adults aged 18 to 64 with BMI between 18.5 and 35 kg/m² and abdominal skinfold thickness of 3 to 8 cm measured by caliper. The study specifically targeted people with localized abdominal fat suitable for reduction, not people with obesity requiring systemic weight loss.
Treatment involved up to four sessions spaced four weeks apart. Each treatment session consisted of 25 to 50 subcutaneous injections into the abdominal area, with each injection delivering 2.4 mL of CBL-514 at a concentration of 2 mg/cm². The maximum dose per treatment was 600 mg. Injections were spaced 2.45 cm apart across the treatment area.
Follow-up visits occurred at 4 and 8 weeks after the final treatment. Subcutaneous fat thickness was measured using ultrasound at four scan points on the abdomen. Fat volume was calculated using these thickness measurements over a defined treatment area of 300 cm². The study also used 3D body imaging to visualize changes.
The primary endpoint was the proportion of participants achieving at least 150 mL subcutaneous fat volume reduction from baseline. Secondary endpoints included proportion achieving at least 200 mL reduction, number of treatments needed, and total fat volume change.
Phase 2 Trial Effectiveness
The trial met its primary endpoint and all secondary endpoints, showing statistically significant fat reduction with CBL-514 compared to placebo.
At the 8-week follow-up (8 weeks after final treatment), 69.6% of CBL-514-treated participants achieved at least 150 mL subcutaneous fat volume reduction, compared to 0% of placebo-treated participants. This difference was highly statistically significant.
For the higher threshold of 200 mL reduction, 60.9% of CBL-514 participants achieved this target at 8-week follow-up, compared to 0% of placebo participants.
The average fat volume reduction was substantial. CBL-514-treated participants showed a mean change in fat volume of approximately 210 mL compared to baseline, which was over 200 mL more than the placebo group. In the per-protocol population (participants who completed all treatments and follow-ups with stable body weight), the mean fat volume reduction exceeded 300 mL.
Importantly, some participants responded to very limited treatment. Of the 28 CBL-514 participants who achieved at least 150 mL fat reduction, 42.9% (12 participants) reached this threshold after just one treatment session. Another 35.7% required two treatments. This means over 75% of responders achieved clinically meaningful results with one or two treatment sessions.
The largest fat volume reduction recorded was over 750 mL in a single CBL-514-treated participant. These reductions occurred with minimal changes in overall body weight. The average body weight change was less than 1 kg in both treatment groups, confirming that fat reduction was localized to the injection area rather than reflecting systemic weight loss.
Representative photographs showed visible reduction in lower abdominal contour, with ultrasound imaging confirming decreased fat layer thickness across measurement points. Notably, participants did not exhibit significant skin laxity after fat reduction, suggesting the skin adapted well to the volume change.
Phase 2 Trial Safety Profile
Safety data from the Phase 2 trial provides important information about the tolerability of CBL-514 treatment.
Nearly all participants (99%) experienced at least one treatment-related adverse event. However, almost all of these were injection site reactions, which is expected with any injectable treatment. The most common adverse events were injection site erythema (redness) in 93.9% of CBL-514 participants, injection site pain in 93.9%, injection site pruritus (itching) in 85.7%, injection site swelling in 83.7%, and injection site warmth in 83.7%.
The severity profile was generally favorable. All participants experienced mild reactions. About half (49%) experienced moderate reactions. Only 9 participants (18.4%) reported severe reactions, all of which involved injection site pain. No placebo participants reported severe reactions.
For participants who experienced injection site pain (63 total across both groups), 60.3% required medication for relief, primarily over-the-counter pain relievers. For the majority (90.5%), pain resolved within one week.
Only two participants discontinued treatment due to adverse events: one due to injection site pain and one due to injection site discoloration. One serious adverse event (peritoneal metastasis, a cancer-related finding) occurred in a CBL-514 participant but was deemed unrelated to study treatment.
Importantly, the severe adverse events associated with deoxycholic acid injections (skin necrosis, ulceration, nerve injury) were notably absent in this trial. Laboratory tests, vital signs, ECGs, and physical examinations showed no clinically significant abnormalities attributed to CBL-514 treatment, suggesting the medication acts locally without systemic effects.
Two participants with pre-existing high cholesterol showed elevated lipid levels at one study visit, with one reporting hyperlipidemia possibly related to treatment. This may reflect fat metabolism after lipolysis, though the small numbers make it difficult to draw conclusions.
How CBL-514 Compares to Other Fat Reduction Options
Understanding how CBL-514 stacks up against existing options helps contextualize what this treatment might offer if it receives approval.
Versus Liposuction
Liposuction remains the gold standard for targeted fat removal, capable of removing large volumes (liters) of fat in a single procedure. However, it requires surgery, anesthesia, significant recovery time, and carries risks including infection, bleeding, contour irregularities, and rare but serious complications.
The Phase 2 trial authors noted that fat volume reductions with CBL-514 were "comparable to that achieved with liposuction," though this claim requires context. Liposuction typically removes much larger volumes than the 200 to 300 mL average seen with CBL-514. However, for people seeking modest fat reduction in targeted areas without surgery, CBL-514 might offer meaningful improvement without surgical risks.
Versus Cryolipolysis (CoolSculpting)
Cryolipolysis is FDA-approved for fat reduction in the abdomen and flanks. Studies have shown varying results, with one reporting average fat volume reduction of 264.8 mL in the abdomen and flanks at 12 weeks after up to 24 treatment cycles over two sessions. Another study showed 39.6 mL reduction in the flank after a single cycle.
Direct comparison is difficult because measurement techniques differ across studies. However, CBL-514 appears to achieve similar or potentially greater fat reduction per treatment session based on available data. The advantage of cryolipolysis is that it requires no injections, while CBL-514 requires dozens of injection points per treatment.
Versus Deoxycholic Acid (Kybella)
Deoxycholic acid is FDA-approved only for submental fat (double chin). It works by disrupting fat cell membranes, causing cell death and subsequent absorption. Multiple treatment sessions are typically needed, and side effects can be significant including nerve damage risk in the submental area.
CBL-514 is designed for larger body areas where deoxycholic acid has not been extensively tested. The mechanism (apoptosis versus membrane disruption) appears to offer a safety advantage based on Phase 2 data, though head-to-head comparisons do not exist.
Who Might Be Candidates for CBL-514?
Based on the Phase 2 trial enrollment criteria and results, potential candidates would include adults who are not significantly overweight (BMI under 35) but have localized abdominal fat that bothers them, people who have achieved weight loss through diet, exercise, or medications but have remaining stubborn fat deposits, individuals seeking fat reduction without surgery or general anesthesia, and people willing to undergo multiple injection treatments for targeted results.
CBL-514 is designed for localized fat reduction, not treatment of obesity. If you need to lose significant overall weight, systemic approaches like GLP-1 medications combined with lifestyle changes would be more appropriate. CBL-514 addresses remaining fat deposits after weight has been lost, or treats localized fat in people who are otherwise at healthy weight.
The trial required participants to maintain stable weight during treatment. This suggests CBL-514 works best when you are not actively gaining or losing weight systemically but want to address specific areas.
Current Status and What Comes Next
CBL-514 is investigational, meaning it is not approved for use outside clinical trials. Caliway Biopharmaceuticals completed Phase 2 trials in 2023 to 2025 and announced plans to begin Phase 3 trials in late 2025.
Phase 3 trials will likely enroll larger numbers of participants, possibly hundreds across multiple sites. These trials will need to confirm the Phase 2 efficacy findings in a larger population and gather more extensive safety data over longer follow-up periods. Regulatory agencies require Phase 3 data before considering approval.
If Phase 3 trials are successful, Caliway would submit for FDA approval, likely in 2026 or 2027. The regulatory review process typically takes 6 to 12 months or longer. Realistically, CBL-514 is unlikely to be commercially available before 2027 at the earliest, and could take longer depending on trial results and regulatory review.
Pricing is unknown. The treatment would likely require a prescription if approved. Insurance coverage for cosmetic fat reduction procedures is generally excluded, suggesting this would be an out-of-pocket expense if it reaches market.
The Relationship to Systemic Weight Loss
It is important to distinguish between systemic weight loss and localized fat reduction. These address different concerns and serve different patient populations.
Systemic weight loss through GLP-1 medications like semaglutide and tirzepatide reduces fat throughout your body by decreasing appetite and calorie intake. You lose weight globally, with reductions in visceral fat (around organs), subcutaneous fat (under skin), and improvements in metabolic health. These medications help with overall weight management and associated health conditions.
Localized fat reduction through injectable treatments like CBL-514 (if approved) targets specific areas of stubborn subcutaneous fat without affecting overall body weight significantly. The Phase 2 trial showed minimal body weight changes despite measurable fat volume reductions in the treatment area.
Some people might benefit from both approaches sequentially. Achieving overall weight loss through systemic approaches first, then addressing remaining localized fat deposits with targeted treatments, represents a logical progression. However, these are different tools serving different purposes.
At Mochi Health, we focus on comprehensive weight management through GLP-1 medications, nutrition counseling, and lifestyle support. While we don't currently offer injectable fat reduction treatments like CBL-514, we help patients achieve significant systemic weight loss that improves overall health. For people interested in learning about weight management options currently available, visit https://joinmochi.com/medications.
Check Your Eligibility
If you want to learn whether GLP-1 treatment is right for you and receive personalized guidance from providers who understand comprehensive weight management, you can start by completing Mochi's eligibility questionnaire. Check your eligibility here: https://app.joinmochi.com/eligibility.
References
Gold, M., Schlessinger, J., Goodman, G. J., Dayan, S., DuBois, J., Ling, Y. F., Sheu, A. Y., Ho, W. W. S., & Chou, Y. C. (2025). Efficacy and safety of CBL-514 injection in reducing abdominal subcutaneous fat: A randomized, single-blind, placebo-controlled phase II study. Aesthetic Surgery Journal, 45(6), 611-620. https://doi.org/10.1093/asj/sjaf032
Shome, D., Khare, S., & Vadera, S. (2022). Efficacy of a novel injection lipolysis to induce targeted adipocyte apoptosis: A randomized, phase IIa study of CBL-514 injection on abdominal subcutaneous fat reduction. Aesthetic Surgery Journal, 42(10), 1154-1164. https://doi.org/10.1093/asj/sjac087
This article is for educational purposes only and should not be considered medical advice. CBL-514 is an investigational medication not currently FDA-approved or commercially available. Consult with healthcare providers about currently available options for fat reduction and weight management appropriate for your individual needs.
Read next
Ready to transform your health?
Unlock access to expert guidance and a weight care plan crafted just for you.

Ready to transform your health?
Unlock access to expert guidance and a weight care plan crafted just for you.

Ready to transform your health?
Unlock access to expert guidance and a weight care plan crafted just for you.


© 2026 Mochi Health
All professional medical services are provided by licensed physicians and clinicians affiliated with independently owned and operated professional practices. Mochi Health Corp. provides administrative and technology services to affiliated medical practices it supports, and does not provide any professional medical services itself.


© 2026 Mochi Health
All professional medical services are provided by licensed physicians and clinicians affiliated with independently owned and operated professional practices. Mochi Health Corp. provides administrative and technology services to affiliated medical practices it supports, and does not provide any professional medical services itself.


© 2026 Mochi Health
All professional medical services are provided by licensed physicians and clinicians affiliated with independently owned and operated professional practices. Mochi Health Corp. provides administrative and technology services to affiliated medical practices it supports, and does not provide any professional medical services itself.